Research
Interface Disorder and Charge Injection into Organic Semiconductors
B.N. Limketkai, M.A. Baldo
Sponsorship: MARCO Materials Structures and Devices Center
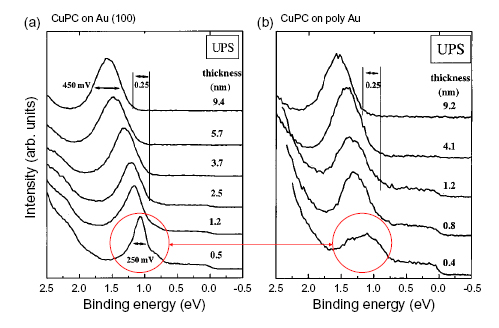
Charge injection dominates the electrical properties of almost every organic electronic device. But the temperature and electric-field dependence of charge injection from metals into films of organic semiconductors has, to date, resisted description by analytic theories.[1] In this project, we examine the effect of structural disorder at the injection interface on the current-voltage (IV) characteristics of organic semiconductors. We find that structural disorder at the injection interface creates energetic disorder with a variance that is typically several tenths of an eV. Thus, structural disorder effectively generates deep interface traps that are observed to dominate the IV characteristics of these materials. For example, ultraviolet photoelectron spectroscopy data taken by Peisert, et al.[2] in Fig. 1 shows the effect of structural disorder on the energetic disorder of the highest occupied molecular orbitals (HOMOs) of copper phthalocyanine on gold surfaces.
Fig. 1. Data of Peisert, et al.[2] showing significant differences in the width of the HOMO of CuPC on flat and rough Au substrates.
Calculations of interfacial disorder
For metal electrodes, interfacial energetic disorder is due primarily to variation in the image charge effect on a rough metal surface. Modeling the metal surface as self-affine, i.e. fractal over a proscribed range of length scales, we find that the standard deviation in the energy levels of the semiconductor is:[3]
where z is the distance of the charge from the metal interface, w is the global rms roughness of the metal interface, q is the electron charge and εrε0 is the permittivity. Most notably, for equally spaced molecular layers, the 1/z2 decay of the energetic disorder yields the ratio of standard deviations of energy levels in the first and second molecular layers:[3]
σ1 and σ2 are the standard deviation of transport states in the first and second layers, respectively.
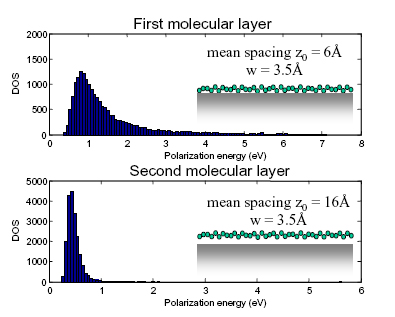
Fig. 2. A simulation of energetic disorder at a rough interface.
This result is independent of material parameters such as the surface roughness. Since the image charge effect at an ideal flat interface scales as 1/z, the 1/z2 dependence of energetic disorder at a rough interface may be understood as the first perturbation of the image charge effect.
There are other causes of energetic disorder at interfaces in addition to the image charge effect. For example, poly(3,4-ethylenedioxythiophene):poly(4-styrenesulphonate) (PEDOT:PSS), a densely charged polymer used frequently as an injection interface, is not expected to exhibit strong image charge effects, but the charge distribution on the PEDOT surface is expected to be disordered. Recently, we have generalized the interfacial disorder calculation to include non-metallic interfaces such as PEDOT. We found that the 1/z2 dependence is also expected at these highly charged polymer interfaces.
Fig. 3. The charged surface of PEDOT is disordered.
Analytic model of charge injection at a disordered interface
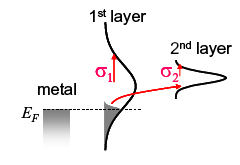
When electrical bias is applied to a disordered metal-organic interface, the voltage determines the quantity of charge trapped in the interface states. The current density is determined by the rate of charge hopping from the interfacial layer to less disordered sites in the second molecular layer.[4] Using the Marcus expression for charge hopping between Gaussian distributions gives a master equation:[3]
where ΔV is the doping-dependent voltage shift, and J0 and V0 are constants. ΔV contains the entire cathode dependence of injection; it is equivalent to the additional voltage required to inject an amount of charge equal to the doped charge present in the boundary layer. The power law slope,
, where λ is the reorganization energy of the molecule. At low temperatures, the decay of energetic disorder gives
Fig. 4. Charge injection dominated by interfacial traps.
Experimental Data
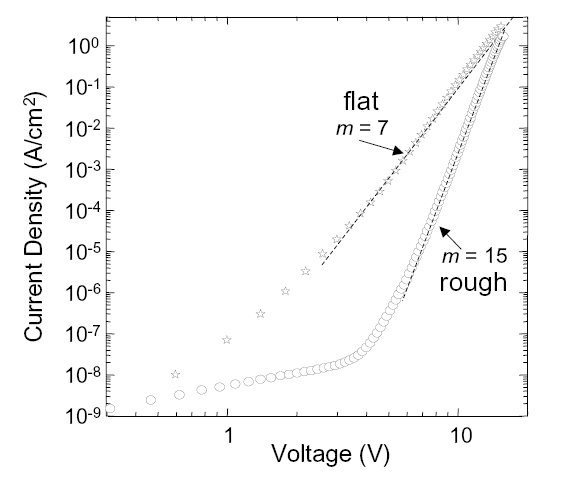
The low temperature IV characteristics of CuPC on flat gold and rough gold are shown in Fig. 5. CuPC on rough gold is a highly resistive contact, exhibiting a power law slope of m = 15. CuPC on flat gold, however, is significantly more conductive, and its power law is only m = 7. Thus, CuPC on rough gold behaves as expected given the large density of traps at its structurally disordered injection interfaces.
Fig. 5. A comparison between the IV characteristics of rough and atomically flat gold injecting contacts to CuPC at T=10K.
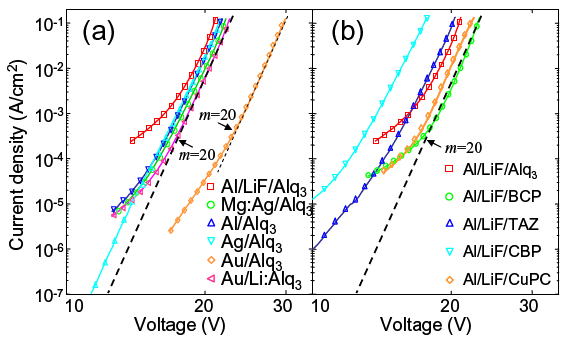
To demonstrate the universality of the model, the IV characteristics of a wide variety of electron injection contacts are shown in Fig. 6. The power law slope is m = (20±1) at 10K, independent of the choice of cathode or organic material, demonstrating that electron injection at these interfaces is not controlled by an energy barrier between the metal and organic semiconductor.
Fig. 6. The IV characteristics at T = 10K for (a) Alq3 interfaces, and (b), a comparison of Al/LiF contacts to Alq3, BCP, TAZ, CBP, and CuPC. All cathodes exhibit similar power law behavior, i.e. J ~ Vm, where m = (20±1).
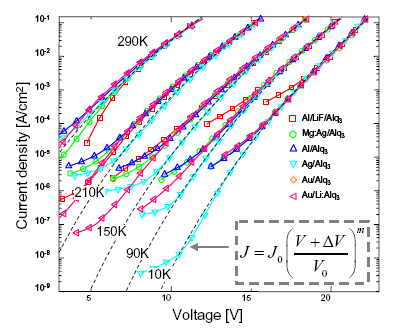
In Fig. 7 we plot the temperature dependence of the IV characteristics for a variety of cathodes on Alq3. If we assume that ΔV contains the cathode dependence, then we expect that all the IV characteristics should be related by rigid shifts in voltage. This is confirmed in Fig. 7.
Fig. 7. The temperature dependence of the IV characteristics of Alq3 interfaces. A rigid voltage shift was applied to the Alq3 characteristics to overlap with the Mg:Ag/Alq3 data. The ΔV=0 fit is shown in dotted lines for each organic material.
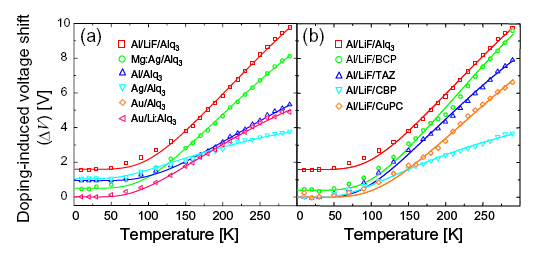
Finally, in Fig. 8, we plot the temperature dependence of ΔV for different cathodes. The temperature dependence of the rigid voltage shift ΔV is observed to fit:[3]
where EA is the activation energy, and ΔV0 is a temperature-independent constant determined by the equilibrium density of charge in the LUMO states at zero temperature. Assuming that ΔV is due to cathode-induced doping of the interfacial states, ND is assigned as the effective doping density.
Fig. 8. The temperature dependence of doping for (a) Alq3 interfaces, and (b), a comparison of Al/LiF contacts to Alq3, BCP, TAZ, CBP, and CuPC. In all cases, the temperature dependence of doping is found to follow Arrhenius behavior.
Conclusion
We have demonstrated that interfacial disorder is an important determinant of the IV characteristics of organic semiconductor injection contacts. Interfacial disorder creates traps, which may be recognized by power law IV characteristics. Since very few practical organic semiconductor devices employ atomically flat injection contacts, we expect that the theory is generally applicable to organic devices.
Relevant publications
- B.N. Limketkai and M.A. Baldo. ‘Charge injection into cathode-doped amorphous organic semiconductors.’ Physical Review B. 71. 085207 (2005)
- M.A. Baldo and S.R. Forrest. ‘Interface limited injection in amorphous organic semiconductors.’ Physical Review B. 64. 085201 (2001)
References
- J.C. Scott. ‘Metal-organic interface and charge injection in organic electronic devices.’ Journal of Vacuum Science and Technology A. 21. 521-531 (2003).
- H. Peisert, M. Knupfer, T. Schwieger, J.M. Auerhammer, M.S. Golden and J. Fink. ‘Full characterization of the interface between the organic semiconductor copper phthalocyanine and gold.’ Journal of Applied Physics. 91. 4872-4878 (2002).
- B.N. Limketkai and M.A. Baldo. ‘Charge injection into cathode-doped amorphous organic semiconductors.’ Physical Review B. 71. 085207 (2005).
- M.A. Baldo and S.R. Forrest. ‘Interface limited injection in amorphous organic semiconductors.’ Physical Review B. 64. 085201 (2001).